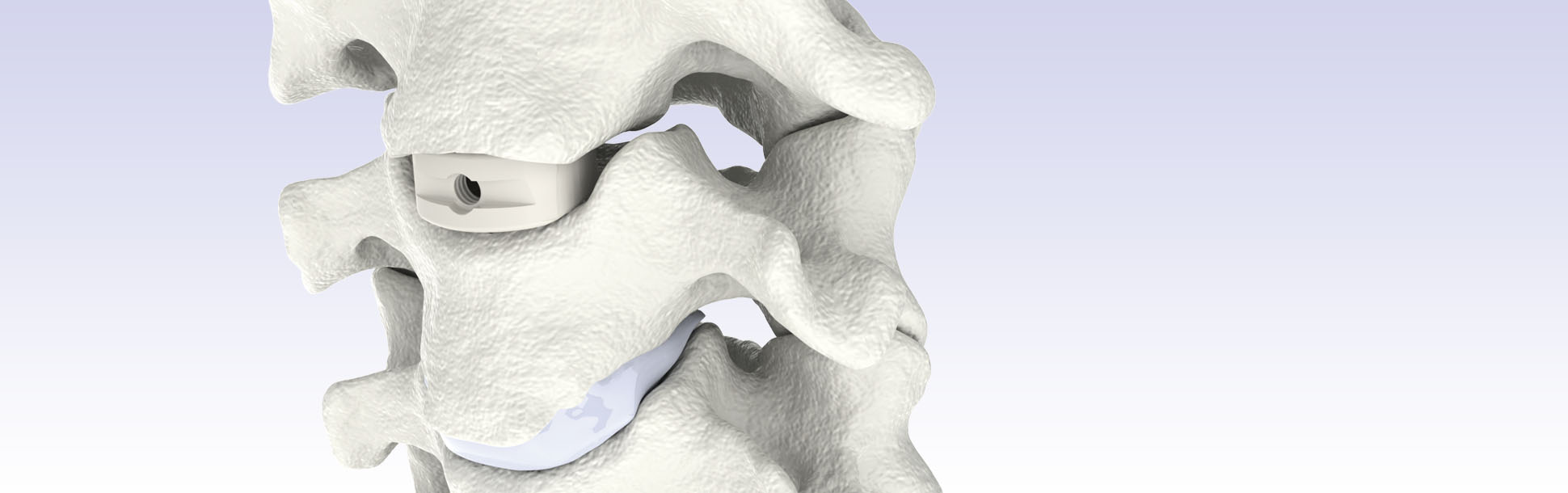
Zervikale interkorporelle Fusion - TRISTAN®
TRISTAN® Zervikale interkorporelle Fusion ist ein Implantatsystem zur langzeitigen Anwendung als Bandscheibenersatz zur anterioren Stabilisierung im Bereich der zervikalen Wirbelsäule von C3 bis C7, bei Patienten, deren allgemeines Skelettwachstum beendet ist. Das System beinhaltet Implantate in verschiedenen Abmessungen, Höhen und Winkelungen, wodurch die einzigartige Anatomie des individuellen Patienten berücksichtigt werden kann.